1. Research Projects
National Health and Medical Research Council (Australia) Early Career Fellow Grant: GNT1127889, A biomimetic prodrug platform to enable oral bioavailability and target lymphatic disease, 2017-2021
2. Representative Research Achievements
Dr. Sifei Han joined the China Pharmaceutical University and started a new research group (co-led with Dr Luojuan Hu) in late 2022. Before that, Sifei worked with Prof Chris Porter and A/Prof Natalie Trevaskis at Monash Institute of Pharmaceutical Sciences, Monash University (Australia) for 7 years focusing on lymphatic drug delivery. Sifei co-led the biopharm team and developed a lymph targeting prodrug strategy that improves drug delivery to pharmacological targets in the lymphatic system, and/or that enhances systemic exposure of drugs such as testosterone and buprenorphine where oral bioavailability is limited due to substantial first-pass metabolism. The technology platform consists of >10 patent families and has been licensed to a clinical stage biotech company PureTech Health (Boston), and sublicensed to Boehringer Ingelheim. A Phase 1 clinical study of LYT-300 (oral allopregnanolone) based on the technology was successfully finished at the end of 2022 and a Phase 2a, proof-of-concept clinical trial is starting in 2023.
Sifei has focused on using delivery approaches to improve the PK/PD profiles of therapeutic agents. A major interest is to enhance drug transport into and through the lymphatic system, which has demonstrated promising utility in a number of aspects. Firstly, the lymph-directing delivery promotes the efficacy of drugs that act on biological targets in the lymphatic system, and it therefore provides opportunities to improve the treatment of related conditions such as (auto)immune disorders, cancer, and acute disease. Secondly, via redirecting drug transport to the lymphatics rather than the blood stream after oral absorption, the prodrug approach also enables the oral bioavailability of drugs that are normally subject to significant hepatic first-pass metabolism.
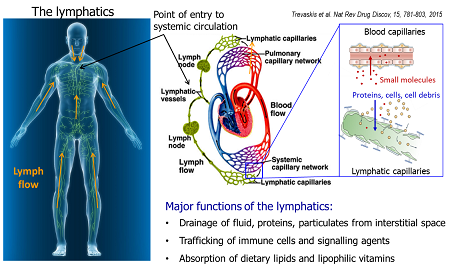
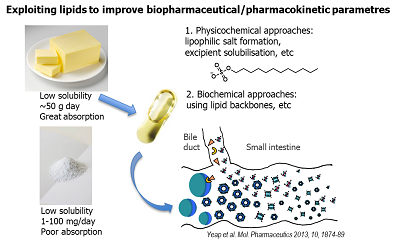
Sifei’s broader interests include using lipid-drug conjugates, ionic liquids and lipid based formulations to enhance the absorption of ‘problematic’ compounds. Sifei is co-inventor of a lymph-targeting technology platform, and a drug candidate from this platform has successfully completed a phase I clinical trial. Sifei’s ongoing research aims to develop an integrated lymph-directing platform for both small and macromolecule drugs. The platform offers the capacity to evaluate drug pharmacokinetic profiles with an emphasis on the absorption, transport and metabolism in the lymphatic system. The platform also utilises the unique advantages of lipids and endogenous macromolecules to optimise drug delivery and pharmacokinetic profiles.
1. Research Projects
National Health and Medical Research Council (Australia) Early Career Fellow Grant: GNT1127889, A biomimetic prodrug platform to enable oral bioavailability and target lymphatic disease, 2017-2021
2. Representative Research Achievements
Dr. Sifei Han joined the China Pharmaceutical University and started a new research group (co-led with Dr Luojuan Hu) in late 2022. Before that, Sifei worked with Prof Chris Porter and A/Prof Natalie Trevaskis at Monash Institute of Pharmaceutical Sciences, Monash University (Australia) for 7 years focusing on lymphatic drug delivery. Sifei co-led the biopharm team and developed a lymph targeting prodrug strategy that improves drug delivery to pharmacological targets in the lymphatic system, and/or that enhances systemic exposure of drugs such as testosterone and buprenorphine where oral bioavailability is limited due to substantial first-pass metabolism. The technology platform consists of >10 patent families and has been licensed to a clinical stage biotech company PureTech Health (Boston), and sublicensed to Boehringer Ingelheim. A Phase 1 clinical study of LYT-300 (oral allopregnanolone) based on the technology was successfully finished at the end of 2022 and a Phase 2a, proof-of-concept clinical trial is starting in 2023.
1.S Han, T Quach, L Hu, SF Lim, D Zheng, NJ Leong, G Sharma, D Bonner, JS Simpson, NL Trevaskis, CJH Porter. Increasing linker chain length and intestinal stability enhances lymphatic transport and lymph node exposure of triglyceride mimetic prodrugs of a model immunomodulator mycophenolic acid. Molecular Pharmaceutics, 2023, in press
2. T Quach, L Hu, S Han*, SF Lim, D Senyschyn, P Yadav, NL Trevaskis, JS Simpson, CJH Porter*. Triglyceride-mimetic Prodrugs of Buprenorphine Enhance Oral Bioavailability via Promotion of Lymphatic Transport. Front. Pharmacology, (2022) doi: 10.3389/fphar.2022.879660
3.Z Fu, S Li, S Han, C Shi, Y Zhang. Antibody drug conjugate: the ‘biological missile’ for targeted cancer therapy. Signal Transduction and Targeted Therapy, 7 (2022), 93
4.S Han*, L Mei, T Quach, CJH Porter, NT Trevaskis*. Lipophilic Conjugates of Drugs: A Tool to Improve Drug Pharmacokinetic and Therapeutic Profiles. Pharm. Research, 38 (2021):1497-1518
5.R Kochappan, E Cao, S Han*, L Hu, T Quach, D Senyschyn, VI Ferreira, G Lee, S Lim, C Nowell, D Bonner, J Mintern, JS Simpson, NL Trevaskis*, CJH Porter*. Targeted delivery of mycophenolic acid to the mesenteric lymph node using a triglyceride mimetic prodrug approach enhances gut-specific immunomodulation in mice. J. Control. Release, 332 (2021), 636-651
6.S Han, T Quach, L Hu, SF Lim, Gracia, NL Trevaskis, JS Simpson, CJH Porter. The impact of conjugation position and linker chemistry on the lymphatic transport of a series of glyceride and phospholipid mimetic prodrugs. J. Pharm. Sci. 110 (2021), 489-499
7.G Lee, S Han, I Inocencio, E Cao, J Hong, ARJ Phillips, JA Windsor, CJH Porter, NL Trevaskis. Lymphatic uptake of liposomes after intraperitoneal administration primarily occurs via the diaphragmatic lymphatics and is dependent on liposome surface properties. Mol. Pharmaceutics. 16 (2019), 4987-4999
8.S Han, L Hu, Gracia, T Quach, JS Simpson, GA Edwards, NL Trevaskis, CJH Porter. Lymphatic Transport of a Triglyceride Mimetic Prodrug in a Large Animal Model: Studies in Greyhound Dogs. Mol. Pharmaceutics. 13 (2016): 3351-3361 (Faculty 1000 recommended article)
9.L Hu#, T Quach# S Han#, SF Lim, NL Trevaskis, JS Simpson, CJH Porter. Glyceride-Mimetic Prodrugs Incorporating Self-Immolative Spacers Promote Lymphatic Transport, Avoid First-Pass Metabolism, and Enhance Oral Bioavailability. Angew Chem Int Ed Engl., 55 (2016), 13700-13705 (Frontispiece )
10.S Han, T Quach, L Hu, A Wahab, WN Charman, VJ Stella, NL Trevaskis, JS Simpson, CJH Porter. Targeted delivery of a model immunomodulator to the lymphatic system: Comparison of alkyl ester versus triglyceride mimetic lipid prodrug strategies. J. Control. Release, 177 (2014), 1-10 (Cover)
Luojuan Hu, Associate Professor (co-PI)
Ph.D. student: Jiazhi Zhang (starting 2022)
Master students: Xiaohua Li (starting 2022), Xiaoxuan Fei(starting 2022), Junyi Pei (starting 2023), Yinyin Yuan (starting 2023), Haodong Chen (starting 2023)
Undergraduate Internship student: Xinyue Zhu (2023)



