On November 25th, 2022, Prof. Yao Hequan and Prof. Lin Aijun’s team from the School of Pharmacy, CPU published their latest research in the top international journal, Angewandte Chemie International Edition (impact factor: 16.823) entitled “Pd-Catalyzed Asymmetric 5-exo-trig Cyclization/Cyclopropanation/Carbonylation of 1,6-Enynes for the Construction of Chiral 3-Azabicyclo[3.1.0]hexanes”. Prof. Yao Hequan and Prof. Lin Aijun are co-corresponding authors, PhD student Li Qiuyu and M.S. student Zhang Yunchu are the co-first authors and China Pharmaceutical University is the sole corresponding institution.
3-Azabicyclo[3.1.0]hexanes, which are rigid molecules containing exquisite sp3-hybridized carbon atoms, are key structural motifs in a myriad of bioactive scaffolds and marketed drugs.[1] For instance, the SARS-CoV-2 main protease inhibitor Nirmatrelvir (PaxlovidTM) was approved by the FDA for the treatment of COVID-19 in 2021 (Figure 1, a). However, the catalytic asymmetric synthesis of these frameworks still poses a formidable challenge. Thus, developing new strategies to assemble structurally diverse chiral 3-azabicyclo[3.1.0]hexanes from readily accessible materials is highly desirable, which will open a new avenue to the previously inaccessible chemical spaces.
Transition metal-catalyzed carbonylation of unsaturated C-C bond with carbon monoxide (CO) is a widely adopted method for accessing functionalized carbonyl compounds. It has been implemented as a key step in the synthesis of natural products and pharmaceuticals. Recently, remarkable progress has been achieved in asymmetric tandem Heck carbonylation. However, they are all initiated from ArX (Csp2-X), and the tandem cyclic carbonylation reaction initiated from propargylic acetate (Csp3-X) remains in its infancy. Yao and Lin’s team disclose a mild and efficient access to chiral 3-azabicyclo[3.1.0]hexanes via a Pd-catalyzed asymmetric 5-exo-trig cyclization/cyclopropanation/carbonylation of 1,6-enynes (Figure 1, b). To demonstrate the synthetic utility of current protocol, various transformation of the products into more structurally diverse 3-azabicyclo[3.1.0]hexane derivatives were conducted (Figure 1, c). This developed 1,6-enynes bicyclization/carbonylation reaction along with the downstream chemistry efficiently enable fast diversification of aza-[3.1.0]bicycle-based building blocks, which is hoped to facilitate their application in drug discovery.
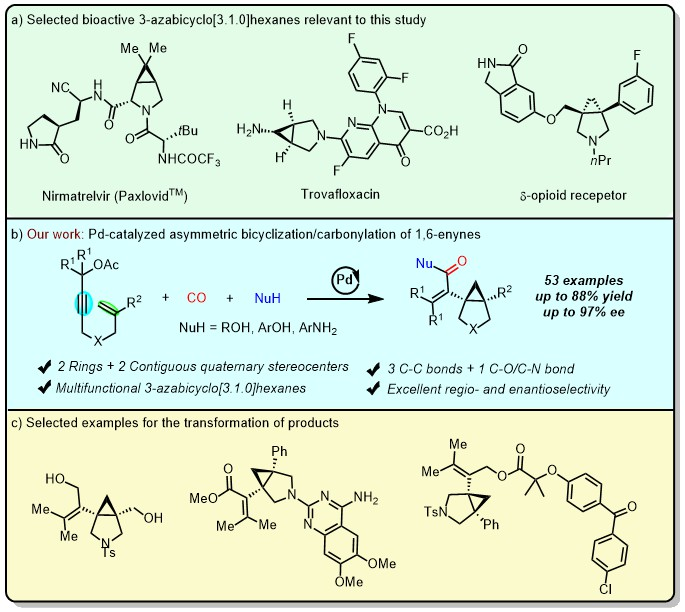
Figure 1. Pd-catalyzed asymmetric bicyclization/carbonylation of 1,6-enynes
This research was supported by the National Natural Science Foundation of China (NSFC22071267) and the Project Program of State Key Laboratory of Natural Medicines (SKLNMZZ202211).
Paper Link:https://onlinelibrary.wiley.com/doi/epdf/10.1002/anie.202211988


